I am analyzing a time course RNA-seq dataset (3 replicates) with 5 timepoints (0h, 1h, 2h, 6h, 24h) and 2 treatment conditions (drug1 and drug2). I want to find out the changes in gene expression patterns across 0-24 hours upon drug treatment. Below is the code I used. My questions:
Why are the logFC values all negative? Am I doing something wrong?
library(edgeR)
library(limma)
x <- read.csv("sample_matrix_rawcount_Tg.csv", header=T, stringsAsFactors=FALSE, row.names=1)
x <- data.matrix(x)
x
Baseline.0h_1 DMSO.1h_1 Tg.1h_1 DMSO.2h_1 Tg.2h_1 DMSO.6h_1 Tg.6h_1
Xkr4 680 650 751 783 542 886 1183
Rp1 2 9 8 22 22 0 1
Sox17 25 40 42 11 53 3 1
...
DMSO.24h_1 Tg.24h_1 Baseline.0h_2 DMSO.1h_2 Tg.1h_2 DMSO.2h_2 Tg.2h_2
Xkr4 1227 1885 1021 1000 477 706 505
Rp1 0 0 0 0 29 0 21
Sox17 0 9 0 0 73 0 14
...
DMSO.6h_2 Tg.6h_2 DMSO.24h_2 Tg.24h_2 Baseline.0h_3 DMSO.1h_3 Tg.1h_3
Xkr4 1071 337 990 1287 930 998 754
Rp1 1 13 0 0 0 0 3
Sox17 1 67 0 0 0 0 44
...
DMSO.2h_3 Tg.2h_3 DMSO.6h_3 Tg.6h_3 DMSO.24h_3 Tg.24h_3
Xkr4 784 566 1125 365 1088 1585
Rp1 0 28 0 16 0 0
Sox17 0 73 0 31 0 0
time<-factor(c("0h","1h", "1h",
"2h", "2h",
"6h", "6h",
"24h","24h",
"0h", "1h", "1h",
"2h", "2h",
"6h", "6h",
"24h","24h",
"0h","1h", "1h",
"2h", "2h",
"6h", "6h",
"24h","24h"))
y <- DGEList(counts=x[,1:27], genes = x[row.names(x)], group = time) #read counts.csv and make table
y$samples
levels(y$samples$group)
dim(y)
[1] 24421 27
head(y$counts)
head(cpm(y))
apply(y$counts, 1, sum) # total gene counts per sample
keep <- rowSums(cpm(y)>100) >= 1
y <- y[keep,]
dim(y)
[1] 3302 27
y$samples$lib.size <- colSums(y$counts)
y$samples
y <- calcNormFactors(y)
y
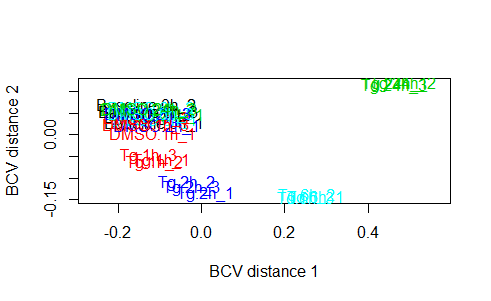
plotMDS(y, method="bcv", col=as.numeric(y$samples$group))
legend("bottomleft", as.character(unique(y$samples$group)), col=1:3, pch=20)
design <- model.matrix(~0+time, data = y$samples)
colnames(design) <- levels(time)
rownames(design) <- colnames(y)
design
0h 1h 24h 2h 6h
Baseline.0h_1 1 0 0 0 0
DMSO.1h_1 0 1 0 0 0
Tg.1h_1 0 1 0 0 0
DMSO.2h_1 0 0 0 1 0
Tg.2h_1 0 0 0 1 0
DMSO.6h_1 0 0 0 0 1
Tg.6h_1 0 0 0 0 1
DMSO.24h_1 0 0 1 0 0
Tg.24h_1 0 0 1 0 0
Baseline.0h_2 1 0 0 0 0
DMSO.1h_2 0 1 0 0 0
Tg.1h_2 0 1 0 0 0
DMSO.2h_2 0 0 0 1 0
Tg.2h_2 0 0 0 1 0
DMSO.6h_2 0 0 0 0 1
Tg.6h_2 0 0 0 0 1
DMSO.24h_2 0 0 1 0 0
Tg.24h_2 0 0 1 0 0
Baseline.0h_3 1 0 0 0 0
DMSO.1h_3 0 1 0 0 0
Tg.1h_3 0 1 0 0 0
DMSO.2h_3 0 0 0 1 0
Tg.2h_3 0 0 0 1 0
DMSO.6h_3 0 0 0 0 1
Tg.6h_3 0 0 0 0 1
DMSO.24h_3 0 0 1 0 0
Tg.24h_3 0 0 1 0 0
attr(,"assign")
[1] 1 1 1 1 1
attr(,"contrasts")
attr(,"contrasts")$time
[1] "contr.treatment"
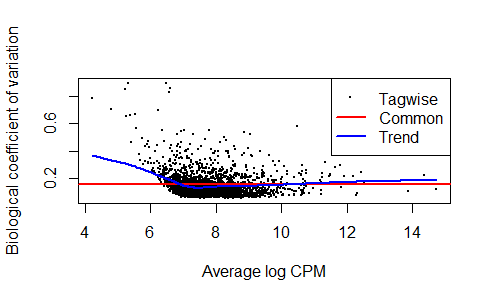
z <- estimateDisp(y,design, robust = TRUE)
sqrt(z$common.dispersion)
plotBCV(z)
plotMDS(z, labels = time)
fit <- glmQLFit(z,design, robust = TRUE)
plotQLDisp(fit)
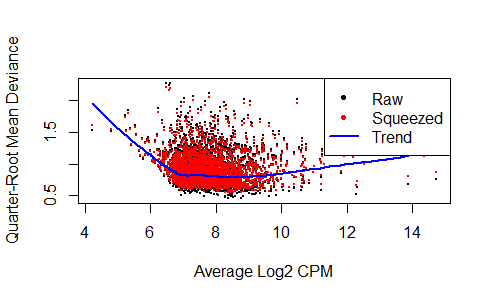
qlf <- glmQLFTest(fit,coef=1:5)
summary(decideTests(qlf))
6h-2h-24h-1h-0h
NotSig 0
Sig 3302
topTags(qlf, n=1000000)
tab2 <- as.data.frame(topTags(qlf, n=1000000))
Coefficient: 0h 1h 24h 2h 6h
genes logFC.0h logFC.1h logFC.24h logFC.2h logFC.6h logCPM F
Ints7 NA -12.26534 -12.27342 -12.35646 -12.28617 -12.25947 7.641854 43991.27
Cs NA -11.58730 -11.55527 -11.53684 -11.59889 -11.56937 8.364561 42149.50
Yme1l1 NA -11.34767 -11.45401 -11.35738 -11.39468 -11.41588 8.533332 40818.42
Elavl1 NA -11.65959 -11.65544 -11.69609 -11.68613 -11.60697 8.271334 40671.67
Pdzd8 NA -11.21080 -11.16845 -11.08415 -11.22524 -11.15235 8.769288 40341.18
Rab14 NA -11.63948 -11.69195 -11.65178 -11.68748 -11.62025 8.271978 39723.03
Ppp6r3 NA -11.48140 -11.49594 -11.50953 -11.50732 -11.40042 8.453886 38766.01
Rnf14 NA -11.90126 -11.87093 -11.85145 -11.84206 -11.89443 8.063408 37941.80
Rabl6 NA -12.42685 -12.48067 -12.42238 -12.39573 -12.36443 7.515743 37101.46
Fam126b NA -12.78592 -12.73044 -12.72118 -12.61550 -12.75967 7.217930 36670.18
Csnk2a1 NA -11.58646 -11.56253 -11.53919 -11.58012 -11.58708 8.362689 36437.87
Ube2i NA -11.95506 -11.96058 -11.93990 -12.06529 -12.02329 7.940364 36216.62
sessionInfo( )
R version 3.6.3 (2020-02-29)
Platform: x86_64-w64-mingw32/x64 (64-bit)
Running under: Windows 10 x64 (build 19042)
Matrix products: default
locale:
[1] LC_COLLATE=English_United States.1252 LC_CTYPE=English_United States.1252
[3] LC_MONETARY=English_United States.1252 LC_NUMERIC=C
[5] LC_TIME=English_United States.1252
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] RColorBrewer_1.1-2 edgeR_3.28.1 limma_3.42.2
loaded via a namespace (and not attached):
[1] compiler_3.6.3 tools_3.6.3 Rcpp_1.0.7 splines_3.6.3 grid_3.6.3
[6] locfit_1.5-9.4 statmod_1.4.36 lattice_0.20-41


Thanks Gordon Smyth , that helped. Also how do I put 24h at the end? I want to plot the observed and fitted values for each gene across timepoints as shown in section 4.8 of the manual. Should I use relevel
time <- relevel(time, ref="0h")?The order of the factor levels makes no difference to the analysis. But you can order the time points if you wish by setting
See
?factor. Usingrelevelwill do nothing because0his already the first level.