I have been trying to annotate the gene symbol for the row name of my data frame H3com using the command:
H9comm$Gene_SYMPOL<- mapIds(org.Gg.eg.db, keys = rownames(H9comm), keytype = 'ENSEMBLTRANS', column = 'SYMBOL')
It pops with an error
Error in .testForValidKeys(x, keys, keytype, fks) : None of the keys entered are valid keys for 'ENSEMBLTRANS'. Please use the keys method to see a listing of valid arguments.
I do not know why, I doubled check that my row names are ensemble transcript ID from chicken, which should match the key.types.
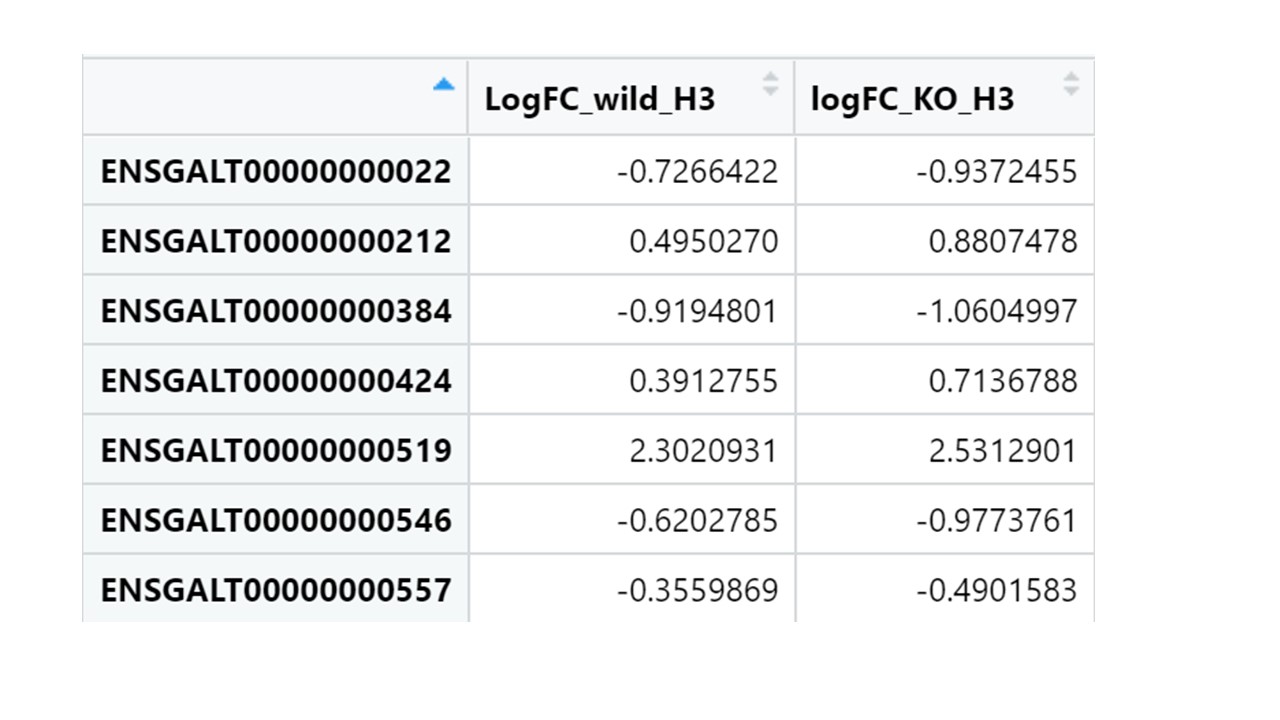
My row names looks like this
Do you think this could be due to any incompitabilities between bioconductor and annotation DB versions ? My Bioconductor version is :
> tools:::.BioC_version_associated_with_R_version()
1 ‘3.15’
and I have installed the annotation DB package from Bioconductor website using
if (!require("BiocManager", quietly = TRUE))
install.packages("BiocManager")
BiocManager::install("AnnotationDbi")
Any help would be appreciated .. Thanks in advance


Thanks James, but looking at my code, it woked for me before very nicely. For me these are ensemble transcript IDs and I am mapping them against a valid key type in the org.Gg.eg.db... so what could be wrong in it ?
Thanks
James's and your code are in essence identical, except for the
keysthat are queried for.Thus:
head(rownames(H9comm))?select(), work for you?mapIds()) work for you when using James's input? Thus:mapIds(org.Gg.eg.db, keys = paste0("ENSGALT", sprintf("%011d", c(22, 212, 384, 424, 519, 546, 557))), keytype = 'ENSEMBLTRANS', column = 'SYMBOL'). If so,the issue is specific to your input, hence point 1.sessionInfo().Thanks and sorry for missunderstanding
What is the output of head(rownames(H9comm))? this produce IDs similar to ones I put in the image above so things transcript Ids like
[1] "ENSGALT00000000022" "ENSGALT00000000212" "ENSGALT00000000384" [4] "ENSGALT00000000424" "ENSGALT00000000519" "ENSGALT00000000546"
Does the code of James, that utilizes select(), work for you? No, gave me the same error:
(Error in .testForValidKeys(x, keys, keytype, fks) : None of the keys entered are valid keys for 'ENSEMBLTRANS'. Please use the keys method to see a listing of valid arguments.)
Does your code (utilizing mapIds()) work for you when using James's input? Thus: mapIds(org.Gg.eg.db, keys = paste0("ENSGALT", sprintf("%011d", c(22, 212, 384, 424, 519, 546, 557))), keytype = 'ENSEMBLTRANS', column = 'SYMBOL'). If so,the issue is specific to your input, hence point 1. No, gave me the same error:
(Error in .testForValidKeys(x, keys, keytype, fks) : None of the keys entered are valid keys for 'ENSEMBLTRANS'. Please use the keys method to see a listing of valid arguments.)
To rule out issues related to package versions, please provide your sessionInfo().
R version 4.2.1 Patched (2022-08-29 r82766 ucrt) Platform: x86_64-w64-mingw32/x64 (64-bit) Running under: Windows 10 x64 (build 19044)
Matrix products: default
locale: [1] LC_COLLATE=English_United Kingdom.utf8 [2] LC_CTYPE=English_United Kingdom.utf8
[3] LC_MONETARY=English_United Kingdom.utf8 [4] LC_NUMERIC=C
[5] LC_TIME=English_United Kingdom.utf8
attached base packages: [1] stats4 stats graphics grDevices utils datasets methods
[8] base
other attached packages: [1] org.Gg.eg.db_3.16.0 AnnotationDbi_1.59.1 IRanges_2.31.2
[4] S4Vectors_0.35.1 Biobase_2.57.1 BiocManager_1.30.16 [7] UniProt.ws_2.37.6 BiocGenerics_0.43.4 RSQLite_2.2.16
[10] edgeR_3.38.4 limma_3.52.2
loaded via a namespace (and not attached): [1] httr_1.4.4 bit64_4.0.5
[3] jsonlite_1.8.0 AnnotationHub_3.5.2
[5] shiny_1.7.2 assertthat_0.2.1
[7] interactiveDisplayBase_1.35.1 BiocFileCache_2.5.2
[9] blob_1.2.3 GenomeInfoDbData_1.2.9
[11] yaml_2.3.5 progress_1.2.2
[13] BiocVersion_3.16.0 pillar_1.8.1
[15] lattice_0.20-45 glue_1.6.2
[17] digest_0.6.29 promises_1.2.0.1
[19] XVector_0.37.1 colorspace_2.0-3
[21] htmltools_0.5.3 httpuv_1.6.5
[23] BiocBaseUtils_0.99.12 pkgconfig_2.0.3
[25] zlibbioc_1.43.0 xtable_1.8-4
[27] scales_1.2.1 later_1.3.0
[29] tibble_3.1.8 KEGGREST_1.37.3
[31] generics_0.1.3 ggplot2_3.3.6
[33] ellipsis_0.3.2 DT_0.25
[35] cachem_1.0.6 cli_3.3.0
[37] magrittr_2.0.3 crayon_1.5.2
[39] mime_0.12 memoise_2.0.1
[41] fansi_1.0.3 PANTHER.db_1.0.11
[43] cellxgenedp_1.1.4 prettyunits_1.1.1
[45] tools_4.2.1 hms_1.1.2
[47] lifecycle_1.0.3 munsell_0.5.0
[49] locfit_1.5-9.6 Biostrings_2.65.3
[51] compiler_4.2.1 GenomeInfoDb_1.33.8
[53] rlang_1.0.6 grid_4.2.1
[55] RCurl_1.98-1.8 rstudioapi_0.14
[57] rappdirs_0.3.3 htmlwidgets_1.5.4
[59] bitops_1.0-7 gtable_0.3.1
[61] DBI_1.1.3 curl_4.3.2
[63] R6_2.5.1 dplyr_1.0.9
[65] fastmap_1.1.0 bit_4.0.4
[67] utf8_1.2.2 filelock_1.0.2
[69] Rcpp_1.0.9 vctrs_0.4.1
[71] png_0.1-7 dbplyr_2.2.1
[73] tidyselect_1.2.0
Thanks, really appreciating any comments.
It indeed looks that you are using the correct input, and since James's code doesn't run for you it points to a problem with your Bioconductor installation.
This problem is confirmed by the output of your
sessionInfo(); it shows that you have a mix of Bioconductor release and development packages installed. This often doesn't work, and this may also cause your problem. (The odd y digits in the x.y.z package versions indicates development branch packages, e.g. AnnotationDbi_1.59.1. You also have installed[org.Gg.eg.db_3.**16**.0], that is also development and not release (which is[org.Gg.eg.db_3.**15**.0]). The annotation packages use the same version numbers as the Bioconductor release, and we are still on version 3.15. Version 3.16 will be released end of this month).You should fix your mixed installation by running
BiocManager::valid()and by following the instructions that come out of it.For your information, my output.